- " HOT SALES "
- About Us
- Lab Products E-Shop
-
Industrial Process Products E-Shop
-
Industrial Process Products-Overall View
>
- BT200 "Dyna-Stream" Batch Series
- VT300 "Dyna-Flyers" Inline Series
- VT310 "Dyna-Core" Ultra High Shear Inline Homogenizer/Mixers Series
- MVHT10-5000 "Dyna-Rotate" Mixing/Blending-Tank/Container Series
- Application from our homogenizer
- Industry Served and Case History
- Advantages from our homogenizer to your industry
-
Industrial Process Products-Overall View
>
- Contact Us
- Reference Corner
- GMP-Good Mixing Practice
|
What do you mean by “mixing”?
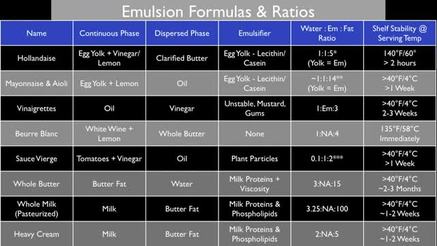
The word “mixing” is used loosely to mean the combination of two or more materials or “phases” to form a so-called “material system”. With such a broad definition, many other terms are used to try to describe particular aspects of mixing, often based on a common understanding in the process industry of what kind of machine is used to generate a particular mixing effect. For instance, blending, homogenisation, dispersion and shearing are all used to indicate certain mixing effects even though strict physical definitions of these effects are not universally agreed. In fluid mixing, components of a material system may be miscible (easily mixed) or immiscible( difficult to mix). In miscible systems, diffusion is the primary means of co-mingling the separate phases, whereas for immiscible systems, mixing energy must be added to overcome the repulsive forces between droplets and particles of one phase relative to the other. In practice, for most material systems, a combination of these effects is required to achieve the typical goals of uniformity and stability. It is worth noting that mixing is involved in many other important physical processes such as fluid structuring and reaction kinetics. Not surprisingly, mixing of some kind is at the heart of most of the process industries. Why is it so important to make small droplets or particles? For many applications, the smaller the particle or droplet, the better the final product. Smaller droplets of one phase within another or particles within a liquid matrix normally gives improved stability and shelf-life, improved product “feel” (texture or mouth feel in foods, or absorption into skin for lotions, for example) and other specialised effects, such as transparency for certain nanoemulsions and nanodispersions. In addition, smaller particles or droplets means increased surface area of contact between the two (or more) phases. This can effect reaction rates between phases, degree of reaction completion and degree of bonding. It is these effects that are generally the most important when we reach the nano-scale so that the true benefits of nanotechnology can be realised. What does “dispersion” mean? We use the term “dispersion” to refer to the type of mixing process involving in particle or droplet size reduction, almost always in immiscible systems or where a powder is being added to a liquid. In general, degree of dispersion is a function of the mixing energy input into a system by applying stresses to the materials, i.e. the higher the energy, the finer the dispersion. For liquid-liquid or solid-liquid systems, there are many and varied ways of applying dispersive mixing energy although each method has a limited range of application and tends to have both advantages and disadvantages. Energy input can be readily compared between methods in kJ/kg but the way in which the material is stressed and relaxed is also critical and is much more dependent on the particular material system being processed. The exact mechanisms of stress application in most dispersive mixers are poorly understood and the complexities of the modelling problem are beyond even the most powerful of today’s computational fluid dynamics models. This means that physical trials are needed for most dispersion applications. Examples of dispersive mixer can be found in the kitchen. The food processor or “bar blender” are typical examples of machines that provide some level of dispersion, used for making emulsions such as mayonnaise, for whipping cream and for reducing the size of solid ingredients in sauces and pastes. What does “distribution” mean? The term “distribution” is generally used to describe a geometrical re-ordering phenomenon that results in blending of two or more materials. Distributive mixing performance is more related to the geometry of a particular mixer with respect to the volume of material being processed and its rheological behaviour than to the amount of mixing energy put into the system. Distribution is probably the type of mixing with which we are most familiar in daily life, from stirring a cup of coffee to mixing a paint colour or making concrete. What do you mean by “viscosity” and “rheology”? The “viscosity” of a substance is a measure of its resistance to flow when a stress is applied. “Rheology” is the study of the flow behaviour of substances, including changes in viscosity, based on their physical composition and response to applied stresses. It particularly relates to complex materials such as muds, crosslinked polymers and certain biological fluids. Common materials exhibiting interesting rheological behaviour include ketchup (syneresis), cornflour starch/water (thixotropy) and rubber (visco-elasticity). For many dispersive mixing processes, shear-thinning and, more unusually, shear thickening are important considerations. These effects relate to the viscosity response of a fluid to applied shear stresses which are common to many types of dispersive mixers. Excellent descriptions of viscosity and rheology can be found on Wikipedia® What is the difference between batch and inline (continuous) fluid mixing? A batch is a discrete volume of material, normally contained and mixed within a vessel. Batch mixers are devices which can be lowered into the vessel from above or protrude into the vessel from below. Inline or continuous mixing is performed by piping the fluid into and out of an inline mixing device. In practice, an inline mixer can be used in three ways: as a true inline , single-pass device; as an inline, multi-pass device (by passing the fluid forwards and backwards through the mixer with a combination of pies and valves), and as a batch recirculating mixer. Inline mixers sometimes need a pump upstream of the mixer to drive material through them whereas batch mixers must self-pump, i.e. be able to push material through their mixing head by themselves. Can you use an inline mixer to recirculate a batch volume? Yes. By piping the outlet of a holding vessel to the inlet of an inline mixer and the outlet of the mixer back to the vessel, fluid will be mixed and recirculated in a similar way to a batch mixer. However, the lack of good distributive mixing in the vessel may lead to incomplete mixing of the batch, so it is common to include a simple impeller in the vessel as an additional device to provide bulk circulation. Explain “emulsification” An emulsion is a mixture of two or more immiscible fluids (phases) and is an inherently unstable material system. Emulsification is the process of mixing the phases together and giving them some stability by reducing the droplet size of the dispersed phase relative to the continuous phase. This typically requires a combination of high dispersive energy from mixing equipment and a suitable chemical emulsifying agent which helps to reduce the surface energy of the droplets to improve the compatibility of the two phases. Generally, the smaller the droplet size, the more stable and useful an emulsion is. Emulsions are typically white in colour but can become transparent if the dispersed phase is small enough and the continuous phase is clear. Most common emulsions use an oily (or lipid) phase and an aqueous phase. They can be creams and pastes, e.g. face cream and machine grease, or low viscosity liquids such as milk. Possibly 20% of dispersive mixing applications are in the field of emulsification. Explain “powder dispersion” The process of mixing solid powder particles into a liquid is commonly called a “powder dispersion” and usually requires a good degree of dispersive mixing with some distributive action. In fact the process comprises three distinct parts; wetting out, dispersion and stabilisation, although these are normally just grouped into the one term. For high volume, rapid powder dispersion it is desirable to separate each powder particle and envelop it in liquid as fast as possible. This allows faster dissolution or stabilisation to occur, for a more efficient process. Larger and higher energy machines that might be expected are often used for this reason and they can be in either batch or inline form. Inline mixers use eductors to feed the powder from a hopper into the fluid stream upstream of a high shear mixer which then does the wetting out and dispersion. One important factor in powder dispersion is the inclusion of air which can be detrimental in certain products such as foods and pharmaceuticals for reasons of increased bacterial growth. The powder itself contains a significant amount of air and this is carried into the fluid unless care is taken to remove it using vacuum prior to addition. Vacuum can also be used on a batch vessel following dispersion to remove entrained air. How do I get rid of lumps or “fisheyes” in my powder dispersion? Certain types of powder dispersions are problematic because they form lumps, also called “fisheyes”, if they are not dispersed quickly. Materials that exhibit this behaviour include many common food and pharmaceutical “hydrocolloid” ingredients such as starch powders, gums, pectin, caseinates, alginates and methylcellulose. If the powder particles are not separated rapidly during the wetting phase of the dispersion, then the outer particles in a clump of material tend to hydrate so that a skin is formed over the clump. This skin is relatively strong and elastic and makes it hard to break the clump down for wetting of the interior particles, resulting in the typical “fisheye”. The answer is rapid and complete wetting out at the point of powder addition using a correctly-sized and operated high shear mixer. The individual particles are therefore separated rapidly and can wet out independently of each other to complete hydration, swelling and gelling over time. How do I mix without aerating the product? This depends on the type of product being mixed. In general, inline mixing is better than batch mixing for reducing air entrainment as the mixer is normally fully flooded in operation. For batch mixing, an inline device can be used in recirculation mode. An exception to the rule of going inline is the use of an eductor with a high shear inline mixer when performing powder dispersion, where air in the powder can be entrained directly into the mixture. Vacuum must be used to remove air from the batch post-mixing in critical applications. Note that in making droplets and particles as small as possible using dispersive mixers, air bubble sizes are also reduced. This makes de-aeration more problematic in the post-mixing phase so it is better to avoid including air in the first place, if possible. How small can I make my particles using mixers and mills? A fluid “mill” is a common term for a type of mixer that is used to reduce the size of solid particles in a liquid. Some kinds of mixer can be used as mills but other mills are specially designed just for this purpose, for example media mills. The ability of a particular mixer to reduce particle size will depend on three things: the material composition of the particle, the fundamental size of the particle and the stress than can be applied by the mill. It is important to note that in fluid mixing, reduction in fundamental size of a particle is not normally achievable for a typical hard material. This is because the fluid “protects” each particle from the applied stresses so that its yield stress is seldom exceeded. A more common ambition is to deagglomerate and/or deaggregate the typical clumps of fundamental particles found in a suspension and then stabilise them at the smallest size possible. Researchers have reported sizes in the region of 50nm but it becomes exponentially harder to achieve much smaller sizes than this due to increased inter-particulate London forces. Maelstrom milling technology in its “Nano & Custom” range can deliver results down to this level with reduced contamination and lower running costs. Can I reverse the mixing process, i.e. separate fluids? Yes. There are both mechanical means and chemical means of doing this using separators and emulsion breakers, for instance. A centrifuge is a common way of separating liquids from solids and flocculants are commonly used to remove particles from waste water. Maelstrom does not supply equipment or chemicals for separation. Which process parameters have the greatest influence on mixer selection? For batch mixing processes, vessel volume and shape, batch volume, fluid viscosity, temperature, pressure, abrasiveness of particles (if any) and desired mixing effect or outcome. For inline mixing, flowrate, viscosity, abrasiveness of particles and desired mixing effect are the most important. |
| ||||||||||||||||||||||||